If we observe the temperature changes of the melting ice or boiling water with the thermometer, then we know that even with continuous heating the temperature of the system never changes.
The heat gained by the system during these processes is known as latent heat. Let’s know about the term latent heat in detail in the below article.
Contents:
What is Latent heat?
Latent heat is the heat exchange by the material at a constant temperature while changing its phase.
It is also known as heat absorbed or rejected by the matter at a constant temperature and is denoted by the symbol ‘L’.
It can be observed during the transition between the solid and liquid state (melting or freezing), liquid and vapor state (vaporization or condensation), and direct transition between the solid and vapor state (sublimation or deposition).
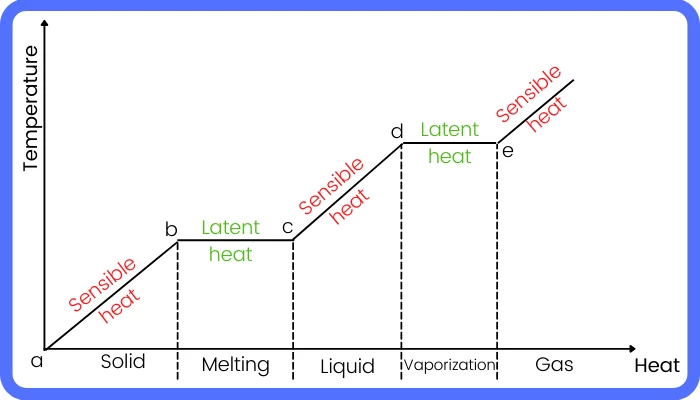
During the phase transition from solid → liquid → gas, the material gains the latent heat to break the intermolecular bond of attraction. While during the phase transitions from vapor → liquid → solid, the molecules of the material lose the latent heat so that the intermolecular forces pull the molecules together that causes a change in phase from vapor to liquid and to solid.
It is sometimes referred to as hidden heat as it never shows the temperature change during its exchange. As it indicates energy, it is measured in terms of a unit of energy i.e. Joules (J).
Specific latent heat is the amount of heat required to change the phase of the unit mass of material. Thus in the SI system, the unit of specific latent heat is J/Kg.
Latent heat equation:
The amount of latent heat required for a substance is given by,
QLatent = L x m
Where
L = Specific latent heat
m = Mass of a substance
Types of latent heat:
Based on the phase change, the latent heat can be classified as follows:
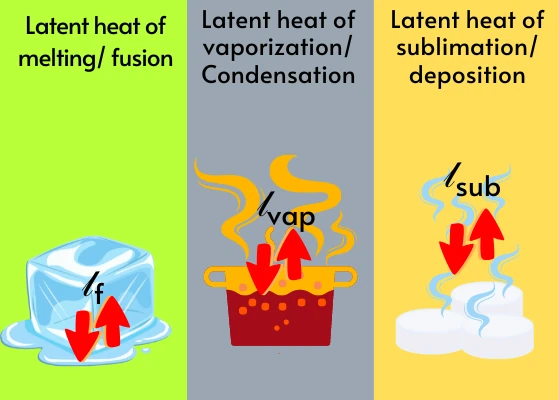
A] Latent heat of fusion/ Latent heat of melting, lf:-
It is the amount of heat gained or rejected by the object to change its phase from solid to liquid or from liquid to solid.
E.g. During the melting, the ice gains the latent heat of melting/fusion and while freezing the water rejects the latent heat of fusion/melting.
The latent heat of fusion is much higher than the sensible heat, that’s the reason why we use ice instead of water to keep beverages cold for a longer time.
Consider the solid material is subjected to heating. Till the melting point, the heat supplied to the material is utilized to raise the kinetic energy of the molecules i.e. to raise the temperature of the material (sensible heating). When the temperature reaches the melting point, the material gains the heat not to increase the kinetic energy but to weaken the intermolecular bonds (Force of attraction between the molecules).
As the intermolecular forces become weaker, the ordered molecules in the solid state become less disordered and get converted into the liquid state.
During the process of freezing, the molecules of the liquid lose the latent heat. It strengthens the intermolecular forces to pull the molecules together to get converted into a solid state.
The heat supplied at the melting point does not increase the kinetic energy of the molecules, hence the temperature of the solid material during the melting remains constant.
B] Latent heat of vaporization/ Latent heat of condensation, lvap:-
It is the amount of heat gained or rejected by the object while changing its phase from liquid to vapor or from vapor to liquid. It is also known as a latent heat of evaporation.
E.g. When water vaporizes, the molecules of water receive the latent heat of condensation/vaporization, whereas when steam condenses into water, the molecules of steam reject the latent heat of condensation/vaporization.
When the liquid reaches boiling point, it gains the heat to break the intermolecular bonds between the liquid molecules. At the boiling point, the heat gained by the liquid is utilized only to overcome the intermolecular forces not to raise the kinetic energy of the molecules.
Thus the molecules become more disordered and it has a significantly less intermolecular force of attraction, which causes the transition of liquid into the vapor state.
During condensation, the vapor molecules lose the latent heat, as a result, the intermolecular forces pull the molecules closer to attain a liquid state.
The value of the latent heat of vaporization is significantly higher than the sensible heat, that’s why steam is used for the heating instead of hot water.
C] Latent heat of sublimation, lsub:-
The latent heat of sublimation is the amount of heat gained by a substance to convert its phase from solid to vapor or it is heat rejected by a substance while converting its phase from vapor to solid.
Sublimation indicates the transition of the phase of the material from solid to vapor without entering into the liquid phase. The reverse of this is known as desublimation/deposition, during which the vapors are converted into a solid state without entering into the liquid state.
E.g. During sublimation, the naphthalene gains the latent heat of sublimation while during deposition, the naphthalene vapors lose the latent heat to the surrounding.
The latent heat of sublimation is equivalent to the sum of its latent heat of melting and the latent heat of vaporization.
Example of latent heat:
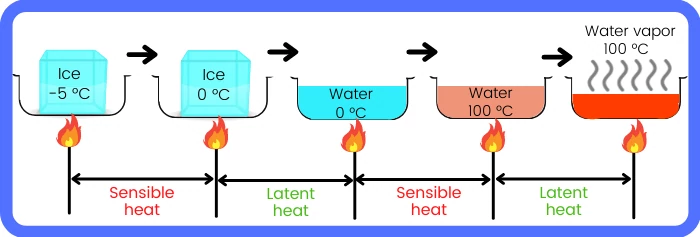
The above figures show the conversion of ice crystals at -5°C to the vapors at 100°C.
The heat supplied for the complete process can be divided into the following parts:-
Ice [-5°C] → Ice [0°C] :- Sensible heat
Ice [0°C] → Water [0°C] :- Latent heat of melting
Water [0°C] → Water [100°C] :- Sensible heat
Water [100°C] → Steam [100°C] :- Latent heat of vaporization
Here at 0 °C, ice gains the latent heat of melting to convert into water at 0 °C.
And at 100 °C, water gains the latent heat of vapourization to convert it into steam.
Latent heat solved problems:
1] Consider 1 Kg of ice at 0° Celsius is heated at atmospheric pressure. Find the amount of heat required to vaporize the ice. (For water, lm = 333.5 KJ/Kg, Iv = 2.25 x 103 KJ/Kg, CP = 4.2 KJ/Kg.K)
Given:
Latent heat of fusion/melting, lm = 333.5 KJ/Kg
Latent heat of vaporization, Iv = 2.25 x 103 KJ/Kg
CP of water = 4.2 KJ/Kg.K
Mass, m = 1 Kg
Solution:-
The heat required to melt 1 Kg of ice is given by,
LMelting = m x lm = 1 x 333.5 = 333.5 KJ
The amount of heat required to heat water from melting point to boiling point at atmospheric pressure is given by,
QSensible = m.Cp. Δt = m.Cp . [TBoiling – TMelting]
As for water, TBoiling = 373 K, TMelting = 273 K,
∴ QSensible = 1 x 4.2 x [373 – 273]
QSensible = 420 KJ
The heat required to vaporize the water at the boiling point is given by,
LVaporization = m x lv = 1 x [2.25 x 103] = 2.25 x 103 KJ
The total heat supplied to the ice to vaporize is given by,
Qtotal = LMelting + QSensible + LVaporization
Qtotal = 333.5 + 420 + 2.25 x 103
Qtotal = 3003.5 KJ
Thanks for reading our article on latent heat, we explained it as thoroughly as possible.
FAQ’s
-
Why latent heat is significant?
The amount of latent heat is much higher than the sensible heat. Thus, it is used for the heating or cooling processes.
-
What factors affect latent heat?
The factors like mass, pressure affect the amount of latent heat required.
-
What are the different types of latent heat?
Based on the phase changes the latent heat is of the following types:
Latent heat of melting
Latent heat of vaporization
Latent heat of sublimation -
Does evaporation cause the release of latent heat?
No, during evaporation, the liquid absorbs the latent heat of evaporation.
-
When is the latent heat considered positive or negative?
The latent heat is considered negative if the material rejects it and it is positive if the material gains it.
-
What mean by latent heat of steam?
It is the amount of heat rejected by the saturated steam to get condense into the saturated liquid.
-
What mean by the latent heat of ice?
It is the amount of heat gained by the ice at melting temperature to change its phase from solid to liquid.
-
What is the effect of latent heat on temperature?
The temperature remains constant during the transfer of latent heat.
-
Is latent heat also a thermal energy?
Yes, latent heat is thermal energy.
-
Are latent heat and enthalpy the same thing?
No, The latent heat indicates the change in enthalpy of the material during the phase change.
See also: