By the use of mercury in a barometer, the total height of the barometer gets reduced as it has a higher density. As well as the mercury has lower vapour pressure, thus its molecules never get evaporated into the evacuated portion and hence it increases the accuracy while measurement.
Before moving to our main question, let’s know about the barometer in brief.
Barometer:
A Barometer is an apparatus that measures atmospheric pressure. It generally consists of an evacuated tube with one end sealed and another end is open which is dipped into the measuring liquid. The measuring liquid inside the container is kept open to the atmosphere.
The conventionally used barometer is shown below,
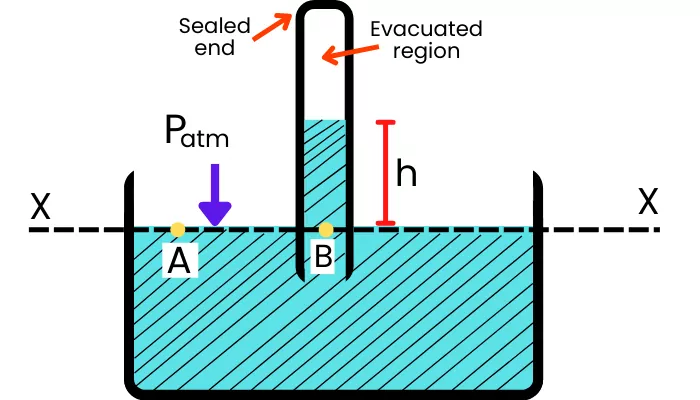
The open surface of the liquid in the container experiences atmospheric pressure and the top surface of the liquid in the tube experiences almost zero pressure.
To find the atmospheric pressure, the barometer compares the pressure made by the liquid column at point B with the pressure on the surface of the liquid at point A.
As shown in the above figure, points A and B lies in the same horizontal line and as the liquid in the above system is stationary, the pressure at point A and B are equal.
`P_{A}` = `P_{B}`
As the surface of the liquid in the container is open to the atmosphere, thus `P_{A}` = `P_{atm}`. Therefore above equation becomes,
`P_{\text{atm}}` = `P_{B}`
Now pressure at point B is given by,
`P_{B}` = Weight of liquid column rise in tube
`P_{B}` = ρ x g x h
Where,
ρ = Density of the measuring liquid
g = Acceleration due to gravity
h = Height of liquid column
By putting this value of `P_{B}` in the above equation of `P_{atm}`, the equation becomes,
`\mathbf{P_{\text{atm}}}` = ρ x g x h
This is the equation to find the atmospheric pressure by using a barometer.
Why mercury is used in barometer?
Following are some reasons that make mercury is suitable for the barometer:-
1] Highly dense mercury requires less height of tube:-
From the above equation of the Barometer, the height of the liquid column can be calculated as
h = `\frac{P_{atm}}{\rho g}`
Thus the relation between the height of the liquid column (h) and density (ρ) is given by,
h ∝ `\frac{1}{\rho }`
From this relation, we can say that the liquid with higher density gives less height of liquid rise in the tube.
As the mercury has a density of 13.6 x 10³ kg/m³ and for the atmospheric pressure at sea level, the minimum rise of mercury in the barometer is given by,
h = `\frac{P_{\text{atm}}}{\rho g}`
h = `\frac{1.013\times 10^{5}}{13.6 \times 10^{3}\times 9.81}`
h = 0.760 m
It means that for mercury, the size of the tube should be greater than 760 mm.
Thus in comparison with some other liquids, mercury gives considerably less height for the liquid rise in a tube of the barometer.
Hence the higher density of the mercury is beneficial to lower the size of a barometer.
2] High accuracy due to lower vapour pressure:-
There is a very high intermolecular force is present between the molecules of mercury. Thus the surface of mercury experiences very fewer amounts of vapour pressure.
Therefore the molecules of mercury never get evaporated into the evacuated portion. Thus it gives higher accuracy for measuring the pressure.
3] Better reading accuracy:-
The mercury never sticks to the wall of the glass tube hence it never wets the glass tube. Therefore it becomes easy to measure the height of the liquid column raised accurately.
Thus mercury is used in Barometer.
Why water is not used in barometer?
The following are the reasons that limit the use of water in a barometer:-
1] Longer tube size due to less density of water:-
At room temperature, water has an appropriate density of 1000 kg/m³.
At sea level, the average atmospheric pressure is 1.013 x 10⁵ N/m².
Thus the approximate minimum height of the water column raised in the barometer tube to predict the atmospheric pressure is given by,
h = `\frac{P_{\text{atm}}}{\rho_{\text{water}}\times g}`
h = `\frac{1.013 \times 10^{5}}{1000\times 9.81}`
h = 10.326 m
Thus this height for the glass tube is too high which cannot fit even into the room.
2] Less accuracy due to high vapour pressure of water:-
Due to the less intermolecular force between water molecules, the vapour pressure on water is very high.
If the water is used in the barometer then due to the very low pressure in the evacuated portion, some water molecules in the tube get evaporate and enter in the evacuated space.
Hence it lowers the accuracy of measuring the atmospheric pressure.
3] Poor reading accuracy:-
The water can stick to the glass of the barometer and thus it wets the glass tube of the barometer. Hence it lowers the accuracy of reading the height of the water column.
Therefore water is not used in Barometer.
Read also: