The main difference between enthalpy vs heat is that enthalpy is a state (point) function that indicates total heat content of the system at a particular state while heat is a path function that indicates energy in transfer.
Let’s take a quick look at each of them.
Contents:
Enthalpy:
Enthalpy is the property that indicates the total heat content of the thermodynamic system. It is denoted by the symbol ‘H’ and expressed in terms of Joules. It is equal to the sum of internal energy and the product of the pressure and volume of the system.
`\therefore H=U+PV`
Where,
H = Enthalpy
U = Internal energy
P = Pressure
V = Volume
As it is difficult to find the total enthalpy of the system, thus change in enthalpy is generally preferred for the calculation which is given by,
`\Delta H = mC_{p}\DeltaT`
Where,
m = Mass of system
`C_{p}` = Specific heat at constant pressure
`\Delta T` = Change in temperature of the system.
Heat:
Heat is the transfer of energy due to the existence of temperature difference between different systems or within the system. The heat always flows from the region of high temperature to the region of lower temperature.
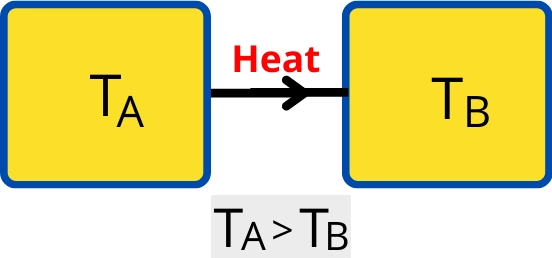
In the case of the objects shown in the below figures as `T_{A}` > `T_{B}`, thus the heat will flow from an object with temperature `T_{A}` to object with temperature `T_{B}`.
The heat is transferred by following three different modes:-
Conduction: The heat transfer through conduction occurs due to the molecular vibrations.
Convection: The heat transfer through convection is due to the conduction and advection (bulk movement of molecules).
Radiation: The radiation indicates heat transfer with the help of electromagnetic waves.
Difference between enthalpy and heat:
| Sr. No. | Enthalpy | Heat |
|---|---|---|
| 1 | The enthalpy indicates the overall heat content of the thermodynamic system. | It indicates energy in transit or flow. |
| 2 | It is denoted by the symbol ‘H’. | It is denoted by the symbol ‘Q’. |
| 3 | Enthalpy is a point function. | Heat is a path function. |
| 4 | The amount of enthalpy losses by the system gets transformed into heat and work. | The amount of heat entering the system becomes part of the enthalpy of the system. |
| 5 | The enthalpy of a system is due to its internal energy, pressure, and volume. | Heat comes into existence due to the presence of temperature differences. |
| 6 | It has an SI unit of a Joule. | It has an SI unit of J/s or Watt. |
| 7 | The process with no enthalpy change is known as the isentropic process. | The process with no heat exchange is the adiabatic process. |
FAQs:
-
Are heat and enthalpy the same thing?
No, enthalpy and heat are the different things.
-
Does heat causes enthalpy to change?
The heat causes the internal energy of the system to change. Due to the fact that enthalpy is dependent on internal energy, heat also affects enthalpy.