The Carnot cycle cannot be realized in practical as it is not possible to attain frictionless working of the components and the perfectly adiabatic processes. As in the Carnot cycle, the isothermal heat transfer is also not possible in an actual engine.
Before moving towards our main question, let’s discuss the basics of the Carnot cycle in brief.
Carnot cycle:
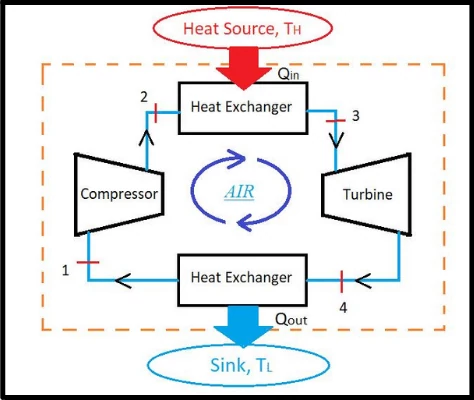
The Carnot cycle is an ideal thermodynamic air standard cycle introduced by the physicist Sadi Carnot.
The cycle consists of four reversible processes which comprise of two isothermal and two adiabatic processes.
Here are the PV and TS graphs for the Carnot cycle,
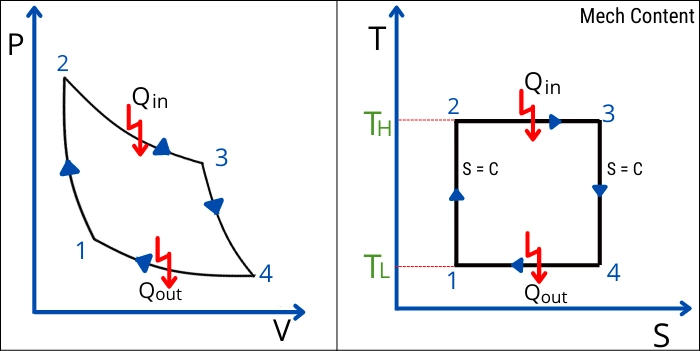
Each process of the Carnot cycle are described below,
Process 1-2: Reversible isothermal compression
Process 2-3: Reversible adiabatic compression
Process 3-4: Reversible isothermal expansion
Process 4-5: Reversible adiabatic expansion
Difficulties for the practical implementing of the Carnot cycle:
To know the difficulties in the practical implementation of the Carnot cycle, it is necessary to understand the necessary conditions for each process.
Process 1-2:- Isothermal compression
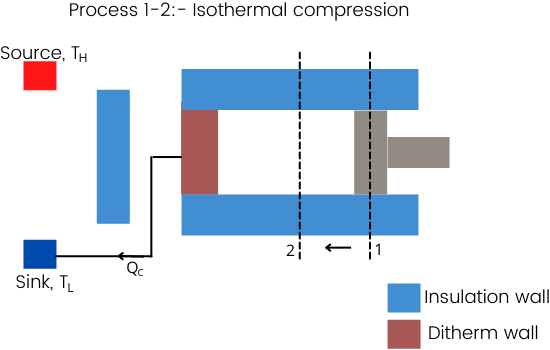
This process 1-2 is an isothermal process at temperature TL.
But because of the compression of the gas, the internal energy of the gas can increase which leads to an increase in temperature. To avoid this, the process of compression has to be done at a very slow speed which is also known as the quasi-static process.
To maintain the constant temperature, the heat generated due to compression has to be liberated to the cold sink and thus the cold sink also has to connect to the diatherm wall.
This quasistatic compression will not give desired output from the cycle.
Process 2-3:- Adiabatic compression
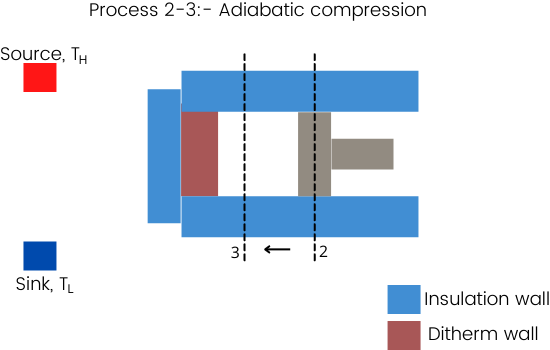
The compression from state-2 to state-3 is an adiabatic process.
To maintain the adiabatic nature of this process, it is necessary to cover the diathermic wall with insulation or the process has to perform at a higher speed to avoid heat losses. And thus it is difficult to perform this process at a faster speed after the quasistatic isothermal compression.
Process 3-4:- Isothermal expansion/heat addition
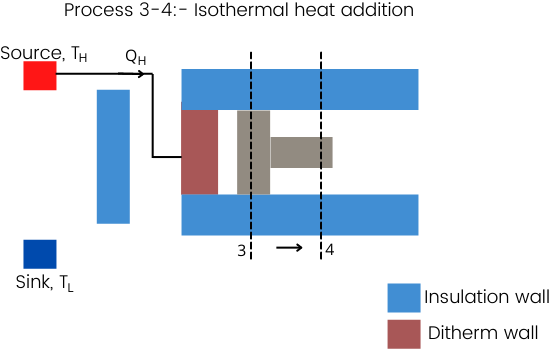
For the isothermal heat addition, the source at a temperature equal to the T3 has to connect to the diathermic wall.
In this process, the heat is added to the gas at a very slow speed without changing the temperature which is possible by the slow expansion of gas.
As the isothermal process is quasistatic, the gas will take much time for this expansion process.
Process 4-1:- Adiabatic expansion
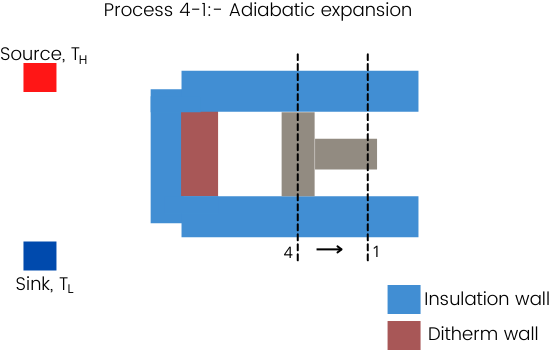
For the adiabatic expansion process, the diathermic wall again has to cover with an insulation wall or the expansion process has to complete at a very faster rate to overcome the heat losses.
From the above discussion about the difficulties for the practical implementation, we can conclude that,
1] For the operation of the Carnot cycle, the diathermic wall has to cover with an adiabatic wall, high-temperature sink, and low-temperature sink in a repeated manner. Therefore it is difficult to continuously cover the diathermic wall with different layers.
2] The isothermal process takes much time for its completion that will take huge time for the completion of a single cycle.
3] The isothermal process has to complete at a very slower rate while the adiabatic processes have to be done at a faster rate and therefore this large variation in speeds makes its working difficult.
4] In actual engines, the isothermal heat transfer can’t be done at considerable speed.
Why carnot cycle cannot be realized in practical?
Here are te some reasons because of which the Carnot cycle is not practically possible:-
1] In the Carnot cycle, all the processes are reversible and it is not possible in the case of actual engines.
2] The isothermal heat transfer is not possible in real heat engines as these processes are quasi-static.
3] The frictionless movement of components is not possible in actual engines.
4] The continuous variation in speed for the isothermal process and the adiabatic process is not possible [The isothermal processes are much slower and adiabatic processes require higher speed].