After a while, the outside tip of a spoon submerged in heated oil gets warm. Similarly, when you start your bike engine, it is at ambient temperature, but after some time has passed, the engine, along with the nearby components, becomes hot.
These are some of the observations in our day-to-day life. In both cases, the heat is supplied at one end of the solid body but it gets transferred to another end of the body.
The heat transferred in solid materials is done by molecular vibration and by the use of free electrons present in solid materials.
Here in this article, we have explained that how heat is transferred in such a solid material.
How is heat transferred in solid materials?
In solid materials, the constituent atoms or molecules are very close to each other and due to the compactness, the energy transfer is done by using the following two modes,
A) By molecular motion:
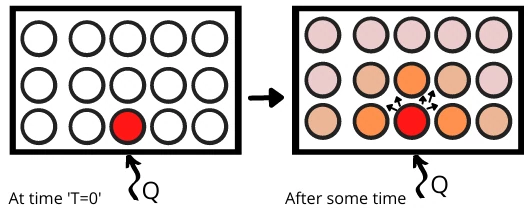
1) When the solid material is exposed to the heat source at one end, the energy is absorbed by the atoms nearest to the surface of the body.
2) By absorbing heat, the kinetic energy of the atoms or molecules gets increased therefore these molecules vibrate at their position.
3) The energized molecules collide with adjacent molecules and transfer the energy to them.
4) Similarly, the kinetic energy of these molecules also gets increased and in the same manner, the molecules also transfer the part of their energies to adjacent molecules.
5) In this way, the energy is transferred from one end to another end in solid materials.
B) By free electrons:-
The free electrons are mostly present in metallic materials. The electrons in such materials can leave the atoms and travel freely inside of the material.
Positive ions are also produced in the substance due to the loosening of electrons. When such materials are exposed to the heat source, the electrons gain heat energy and moves faster with higher energy level.
When such electrons hit the positive ions, the energy gets transferred to these atoms and therefore kinetic energy of these atoms gets increased. In this way, the free electrons help to rapidly transfer heat in solid materials.
Relevent articles: